A custom modular GMP suite of workstations will be used for Cell Therapy. The customer, based in the South of England, is working on a novel new approach to T Cell therapy whereby they are building up a stock of T Cells derived from healthy donors which can be administered to cancer patients.
The system consists of three sections. Section 1 is a H135 HEPA workstation, Section 2 is aH155 GMP workstation, and Section 3 is a refrigerated H135 HEPA workstation. Each section will be attached through a shared airlock.This is represented in the illustration below:
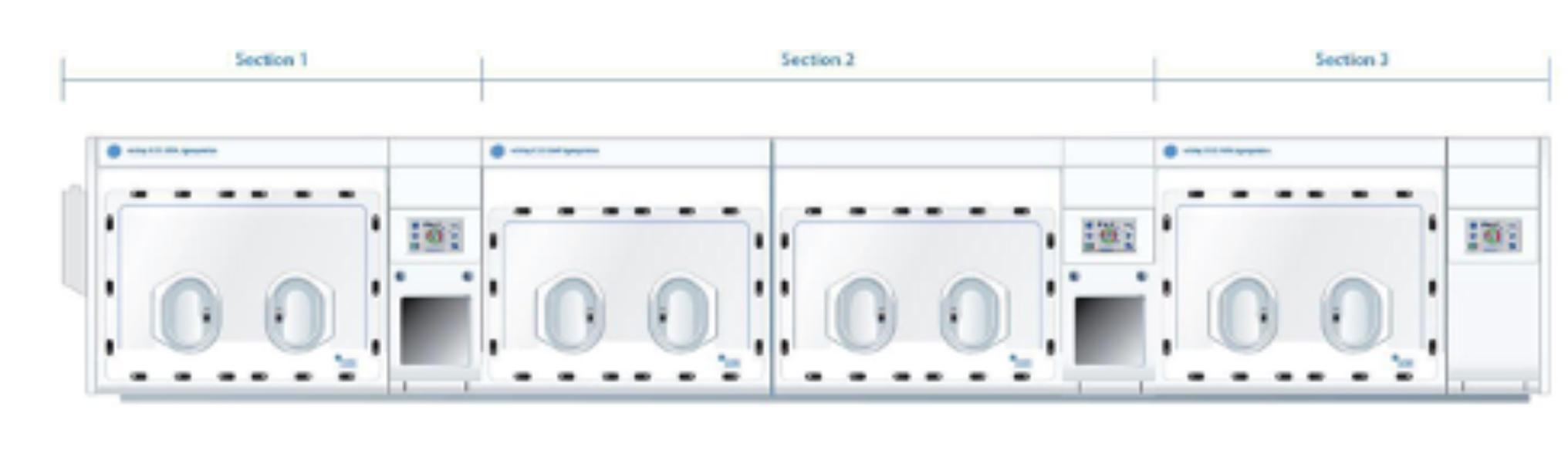
Section 1 is an H135 HEPA workstation used for hypoxic incubation. This workstation is a two-glove system designed for a single operator, and will create a controlled atmosphere, allowing users to define the required oxygen concentration, carbon dioxide concentration, temperature,and humidity. This workstation will be fitted with a sterile steam humidification system as well as an automated dehumidification system, allowing fine control of humidity levels. This workstation has our standard HEPA (turbulent air filtration system) which will achieve Grade A conditions of non-viable particulate at rest and Grade C conditions in operation, as defined in Annex 1 of the EU Guidelines to Good Manufacturing Practice: Medicinal Products for Humanand Veterinary Use. This section of the workstation suite is intended to be used for cell culture at 37°C±1 °C.
Section 2 is an H155 GMP workstation. This workstation is a four-glove system designed to allow two operators to work side by side, and will create a controlled atmosphere, allowing users to define the required oxygen and carbon dioxide concentrations. This workstation will be fitted with an automatic dehumidifier. This workstation will also provide sterile unidirectional airflow which will achieve Grade A conditions of non-viable particulate both at rest and in operation. This section of the workstation suite is intended to be used as a ‘processing area’ for‘high-risk’ manipulations, in which the product containers are open and thus the product may have direct contact with the surrounding atmosphere.
Section 3 is a refrigerated H135 HEPA workstation intended to be used for refrigerated media storage at approximately 8 °C±2°C.
The three workstations are connected through shared airlocks, allowing items to be passed from one section to another, into the sections from outside, or out of the sections from the inside. The airlock connecting Section 2 and Section 3 will also be capable of being sterilised with the Steris M100 vaporised hydrogen peroxide (VHP) system to allow decontamination of items passed into the workstations from the background room.You will note that these airlocks are larger than normal. They are 30 cms high, 30 cms deep and 32 cms wide.The design is such that Corning Cell STACK cell ware can pass through each opening, which is what this customer will be using.
Taken as a whole, the workstations are intended to be used as a clean-air system in processes following Good Manufacturing Practice (GMP). It provides a Grade A air cleanliness zone using sterile unidirectional airflow and provides particulate control in the rest of the sterile envelope using turbulent airflow filtration systems. These air filtration systems combine with positive operating pressure and physical isolation to provide highly effective product protection. The workstations are also designed to be decontaminated using VHP, with the intention that the system will be closed and decontaminated prior to use and will maintain sterile conditions throughout the manufacturing process via use of an airlock system with independent VHPcapability.
Due to being a completely closed re-circulating system (i.e., not requiring inlet air from the occupied room), this workstation can be housed in a Grade D cleanroom during GMP-compliant use. Samples maybe introduced, manipulated, examined, and removed without any loss of environmenta conditions.
As part of the development process, we produced a 45-page Functional specification document to answer the customers User Requirement Specification (URS). We can validate the HEPA system in both the HEPA and GMP workstations. Additionally, we offer a validation package which covers the below tests (whereapplicable)
Example I QOQ sections
- Section1–GeneralTests(touchscreen,airlockfunction,portholesetc)
- Section2 –DOP+ FilterFaceVelocity (Test1+2)
- Section3–Pressuretesting(Test3)
- Section4 –Smokesticktests(Test4)
- Section5–MicrobialAirSampler(Test 5)
- Section6– UKASTempmapping (Test 6)
- Section7 –RH% Independentsensor(Test7)
- Section8 –Aerosolrecovery time(Test 8)
- Section9–Leaksoftwareroutine(Test9)